Public / Direct Reporting
Global solution with simple, self-service interface enabling reporting at source via corporate & product websites with touchless processing
Standardise data across all intake routes, increase efficiencies, and accelerate safety insights
Transform the capture, management & processing of adverse event, drug safety & product quality complaint data

Provide a dynamic experience with standard data export

Streamline processes, automate transfer and reduce follow up

Reduce case processing and management effort for vendors and safety teams
Provide an omni-channel user experience to enable patient, caregiver, and HCP reporting via corporate & product websites
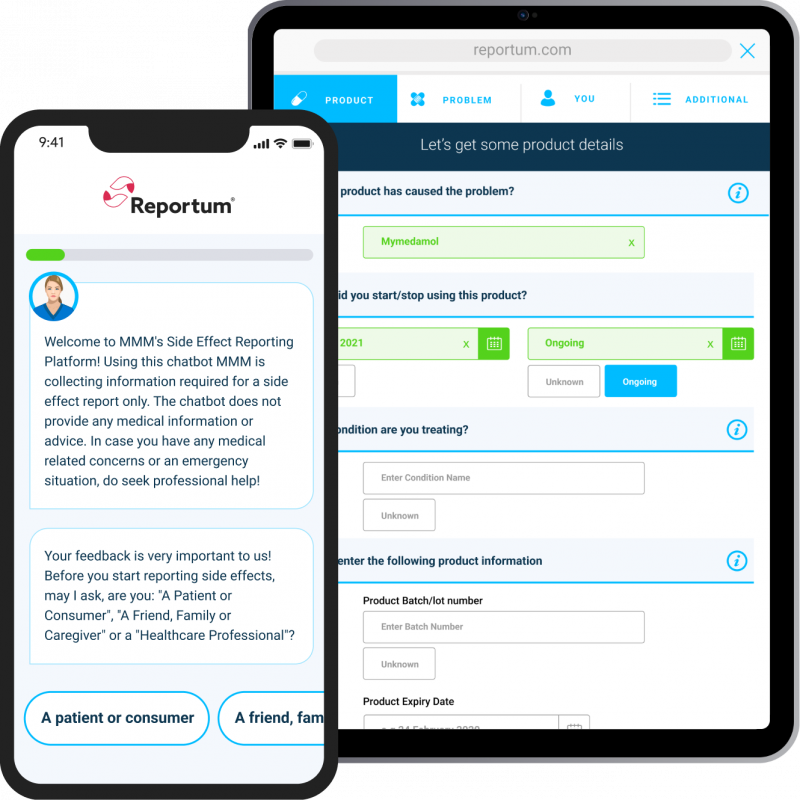
Intuitive, empathy-driven interface
Simple, local language system connecting with all types of reporters supported by dynamic questioning to guide users and ensure key data is collected right first time across all intake channels.
Standardised case data
Consolidate the full process onto a single platform for all sources, with instream coding and consistency checks.
Any device, any time
Highly available cloud infrastructure with online/offline capability ensures data can be entered anywhere, at any time.

Highly configurable platform that enables deployment in line with company branding, workflows and portfolio requirements.
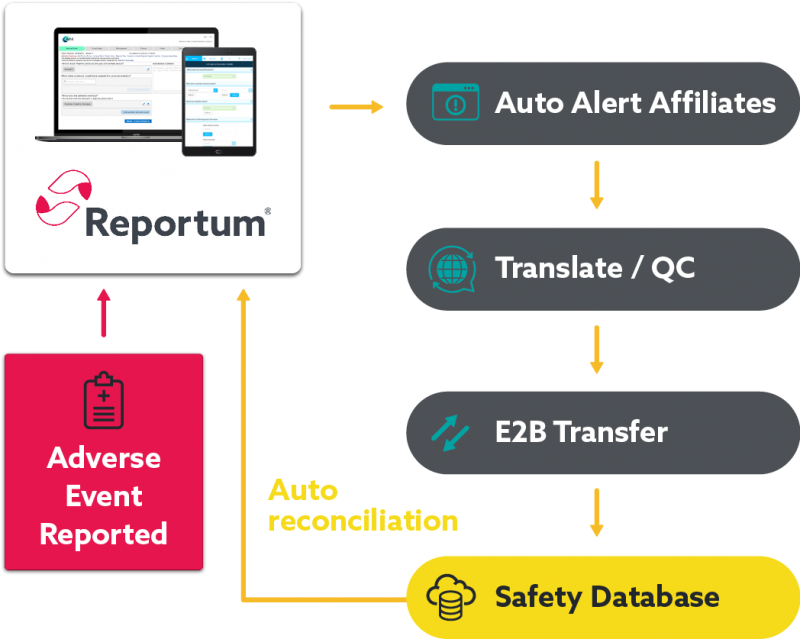
Seamlessly transfer data between systems to optimise the end-to-end pharmacovigilance process.
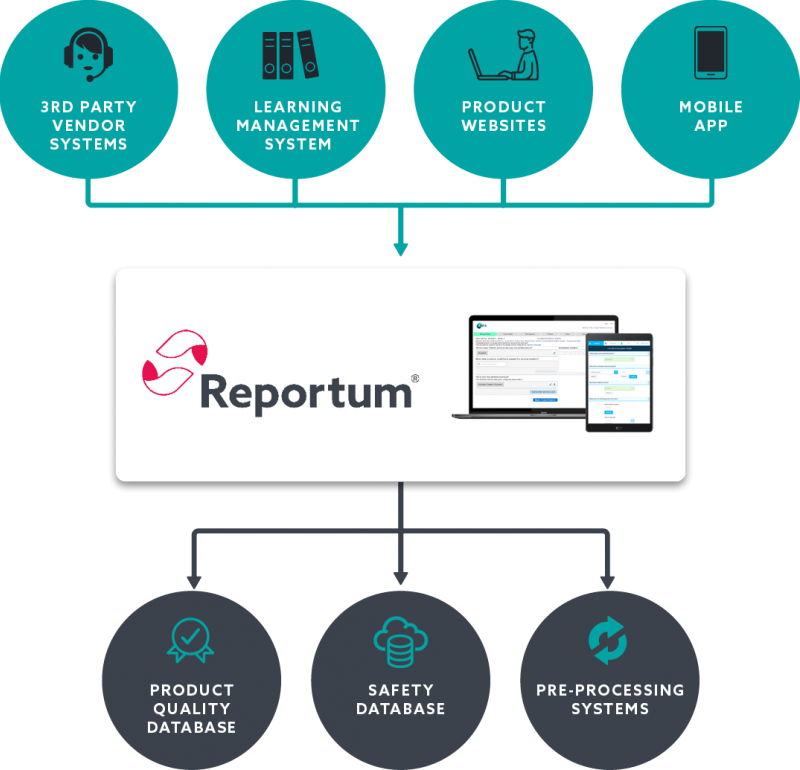
Implement a centralised solution across all intake routes to increase data visibility and drive faster signal management.
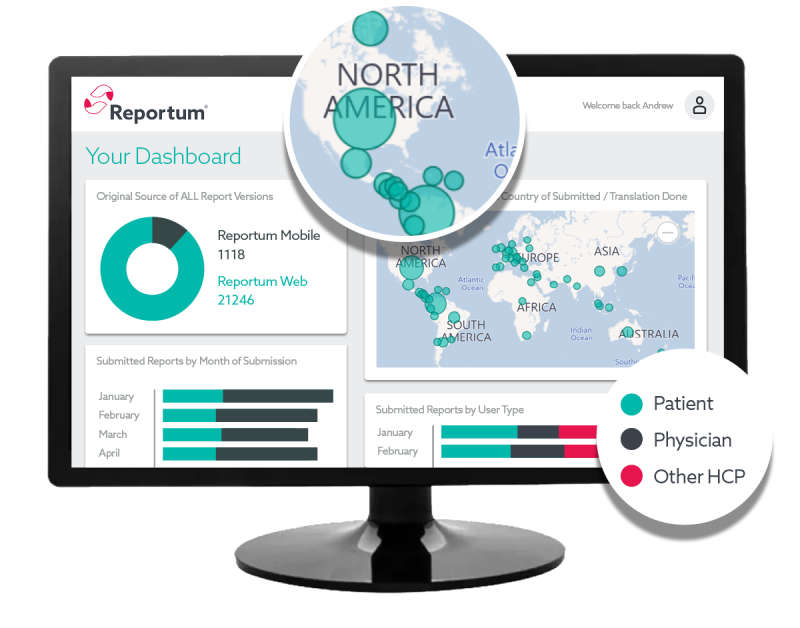
Preconfigured and verified out-of-the-box platform for rapid implementation and cost-effective deployment

Reportum is a fully configurable drug safety reporting solution, supporting adverse events and product quality complaint intake across all channels, languages, and reporter types
Global solution with simple, self-service interface enabling reporting at source via corporate & product websites with touchless processing
Multi-lingual, intuitive interface with online/offline capability to facilitate quick & easy reporting of key information
Expert user interface and workflow, reducing time-per-report and reconciliation activities, ensuring complete data transfer to downstream systems
Guided collection, translation and automatic routing, including data redaction, resulting in improved data privacy and compliance
Program specific solution for nurses or case managers enabling standardized and complete data capture across multiple third-party service providers
Unified solution for required training & certification of HCPs, and the capture of adverse events including targeted questionnaires
To find out more or request a demo fill in the details below: