Dr Andrew Rut February 2022
Following on from his recent article on the importance of communicating clear, meaningful safety information, in this article Dr Andrew Rut discusses the need for proactive capture of safety information following product launch so that a complete safety profile can be rapidly developed and communicated.
What if those of us who are practicing physicians said to the patient in front of us; ‘…and just to let you know you are the five hundred and first patient who will be taking this medicine for longer than 6 months… oh and you’ll be taking it for the rest of your life’? Patients may hesitate to take the medication.
We know that the benefits and risks of medication at the individual level is governed by many factors, including genetics, lifestyle, the presence of chronic diseases, and concomitant medications. However, the nature of clinical trials means they are conducted in highly selective populations with narrow definitions of ‘disease’ and, even for chronic conditions, for a relatively limited duration.
In contrast, many medicines are intended to be used for years, if not a lifetime, across a demographic mix significantly broader than the original trial populations. Therefore, the boundaries of knowledge at market authorisation are narrow particularly with respect to potential risks; only limited information on the true safety profile is available.
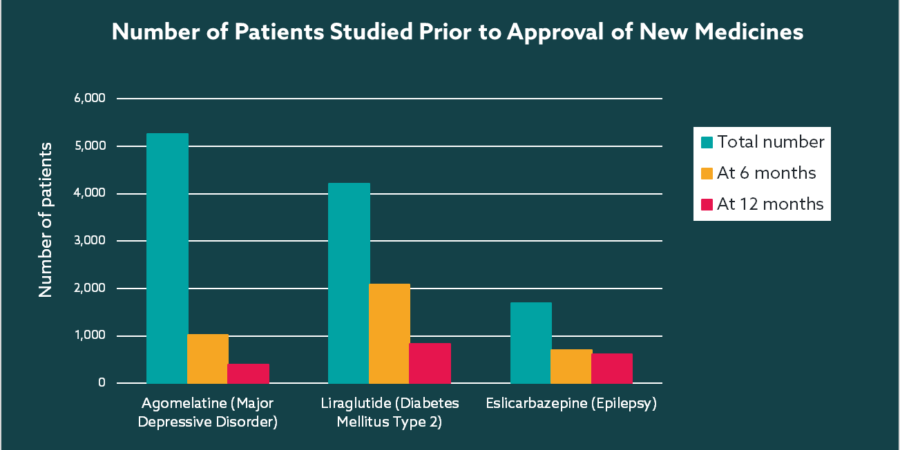
If we look at the number of subjects exposed for meaningful periods of time during late phase trials, the numbers are often worryingly small. A study into the number of patients who had been administered medicines at the time of approval by the European Medicines Agency uncovered a dramatic drop off over time for many long-term chronic conditions, including epilepsy, diabetes, depression (see below)1. Just several hundred subjects studied for 6 or 12 months means long-term safety profile is unknown and the study population is but a tiny representation of the broader target population. Such limited exposure leaves significant gaps in understanding of the true safety profile at the time of launch, therefore it is no surprise that the authors conclude ‘For medicines intended for chronic use, the number of patients studied before marketing is insufficient to evaluate safety and long-term efficacy’.
Case in point: vaccine hesitancy
Vaccines have been thrown into the spotlight as a result of the COVID-19 pandemic, particularly as some of them use completely novel technology. The speed of development and lack of long-term data is one of the main reasons provided by those who describe themselves as ‘vaccine hesitant’2.
A survey conducted by the UK Office for National Statistics3 as the first vaccines were being approved found the most common reasons for vaccine hesitancy were:
- feeling worried about the side effects (52%)
- wanting to wait and see how well the vaccine works (52%)
- feeling worried about the long-term effects on their health (46%)
Acknowledging people’s concerns and then showing a planned and thoughtful approach to understanding the long-term sequelae of vaccination can only have a positive impact trust in the healthcare system.
Proactive knowledge expansion post-launch
Recognising the limitation of knowledge at launch, Pharma must be proactive in gathering data on its products in the post-approval phase. Clearly there is a huge drive to gather ‘real world data’ over years through non-interventional programs, this can be complemented through spontaneous event capture to identify those rare, unusual effects across the diversity that is humanity. There can be no single source of data; ideally just make it really easy to report and capture events in real time from the source; i.e. the patient, their caregiver or healthcare professional.
Only with systems that focus on the source, will we accelerate acquisition of data that is both relevant and routed fast for analysis so that that accurate and up to date information can be communicated as it becomes available. No patient wants to be left in any doubt that their data is valued, captured as they intended, and acted upon.
In his next article Dr Rut will explore why so many adverse events go unreported and what industry can do to make safety reporting easier and more accessible.
References
1. Number of Patients Studied Prior to Approval of New Medicines: A Database Analysis
2. Assessing the long-term safety and efficacy of COVID-19 vaccines
3. Coronavirus (COVID-19) weekly insights: latest health indicators in England, 18 December 2020









