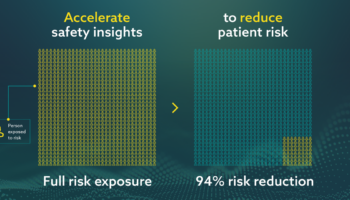
This whitepaper, developed in partnership with Sidley Austin LLP, provides an in-depth analysis of the impact of the COVID-19 mass vaccination campaigns on pharmacovigilance teams, including:
- The mass influx of adverse events reported within a short period of time
- The need for rapid signal evaluation and impact assessments
- Increased liability risks given the prominence and scale of the vaccination programs
Download this whitepaper to learn why pharmacovigilance departments for all pharmaceutical companies, regardless of whether they are a COVID-19 manufacturer, must be prepared for the anticipated surge in adverse event reporting.